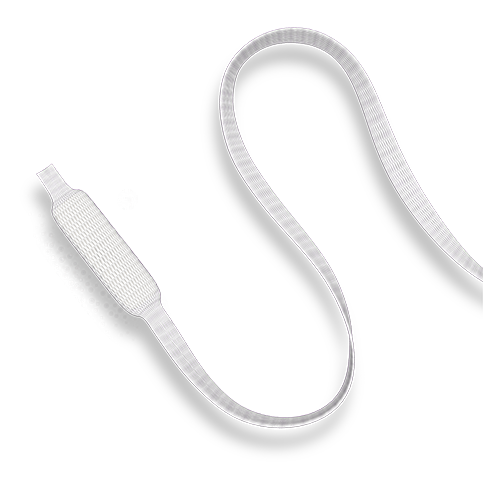
CERVIDIL® (dinoprostone, 10 mg) is a vaginal insert approved to start and/or continue the ripening of the cervix in pregnant women who are at or near the time of delivery and in whom there is a medical reason for inducing (bringing on) labor.
For the first two (2) hours following insertion, you should remain lying down. If you sit up or walk after the first two hours, you should be careful to ensure the insert remains in place. While CERVIDIL is inserted, your doctor will carefully monitor your progress and your baby’s well-being and will determine when the insert should be removed.
Important Safety Information about CERVIDIL
CERVIDIL should only be inserted by a trained healthcare professional in a hospital setting appropriate for childbirth.
WHO SHOULD NOT BE GIVEN CERVIDIL?
You should NOT be given CERVIDIL if you have:
- Experienced an allergic reaction to prostaglandins (certain hormone-like substances)
- Experienced unexplained vaginal bleeding during your pregnancy
- Already started receiving drugs to induce labor
- Given birth six or more times in your lifetime
You should also NOT be given CERVIDIL if your doctor has determined that:
- Your baby is in distress and needs to be delivered urgently
- Your baby may be too large to fit through your birth canal (“cephalopelvic disproportion”)
- Drugs used to induce labor are not appropriate for you or that prolonged contraction of your uterus may be harmful to you or your baby such as if you have had a previous cesarean section or major surgery on your uterus.
What are the most serious risks associated with the use of CERVIDIL?
The induction of labor has been associated with an increased risk of a disorder of abnormal clotting of the blood that results in excessive bleeding immediately after birth (“disseminated intravascular coagulation” or DIC). The risk is higher in women over age 30, those with complications during pregnancy, and those whose pregnancy has lasted longer than 40 weeks.
In rare cases, the use of CERVIDIL has been associated with an increased risk of a life-threatening event to the mother called “amniotic fluid embolism.” The cause of amniotic fluid embolism is not well understood but it is believed that some amniotic fluid or other substances can get into your bloodstream and start a severe reaction that can cause heart and lung collapse.
What should I discuss with my Doctor before labor induction begins or CERVIDIL is given?
As you would throughout your pregnancy, be sure to tell your doctor about all prescription or over-the-counter medications you are taking. Before CERVIDIL is given, be sure you have told your doctor about all your current and past medical conditions, including:
- If your water has broken
- Any unexplained vaginal bleeding during pregnancy
- All uterine surgeries, especially previous cesarean section
- A history of contractions lasting more than 2 minutes
- Glaucoma
- Asthma, even if you had childhood asthma and have had no asthma attacks as an adult
What are the most common side effects of CERVIDIL?
The most common side effects associated with the administration of CERVIDIL are contractions occurring at a rate faster than normal (tachysystole) and signs that the baby is exhausted or in distress (uterine hyperstimulation). In clinical trials, these effects occurred alone or together in less than 1 in 20 women who were given CERVIDIL.
In clinical trials, fever, nausea, vomiting, diarrhea and abdominal pain were noted in less than 1 in 100 women who were given CERVIDIL.
This is not a complete list of possible side effects.
If you experience an adverse event please discuss it with your doctor.
You are encouraged to report negative side effects of prescription drugs to the FDA.
Visit www.fda.gov/medwatch/ or call 1-800-FDA-1088.
People depicted in images are models. Images used for illustrative purposes.
Please see full Prescribing Information.
REFERENCES: 1. CERVIDIL [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc. 2. 2018 FDA Orange Book. https://www.accessdata.fda.gov/scripts/cder/ob/default.cfm. Accessed August 17,
2015. 3. The American College of Obstetricians and Gynecologists. Frequently Asked Questions: Labor, Delivery, and Postpartum Care. http://www.acog.org/Patients/FAQs/Labor-Induction. Published 2017. Accessed March 5, 2018.