Intended for U.S. Healthcare Professionals
Expecting MomsProven to Work
In clinical trials, CERVIDIL provided successful ripening over a 12-hour period1
Proven to Work
In clinical trials, CERVIDIL provided successful ripening over a 12-hour period1
A single dose of FDA-approved CERVIDIL successfully ripened the cervix in the majority of patients in clinical trials*
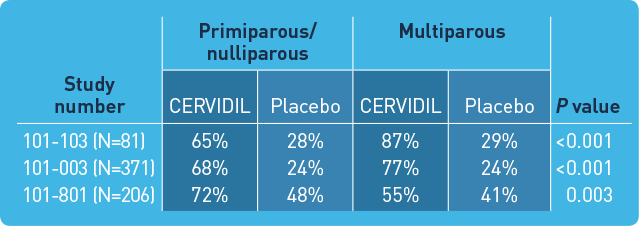
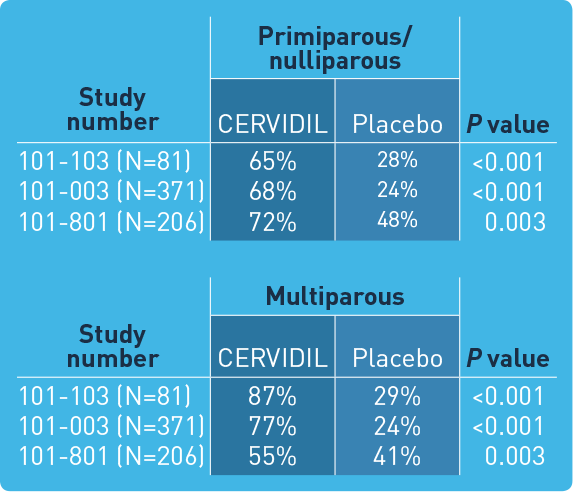
Study design: CERVIDIL was studied in 3 randomized, double-blind, placebo-controlled clinical trials in which 658 women were entered and 320 received active therapy. Studies 101-003 (N=371) and 101-103 (N=81) involved the vaginal pessary alone, whereas study 101-801 (N=206) used the vaginal pessary inserted into a knitted polyester retrieval system.
*Treatment success was defined as Bishop score increase at 12 hours of ≥3, vaginal delivery within 12 hours, or Bishop score at 12 hours of ≥6.
An Effective Way to Ripen1
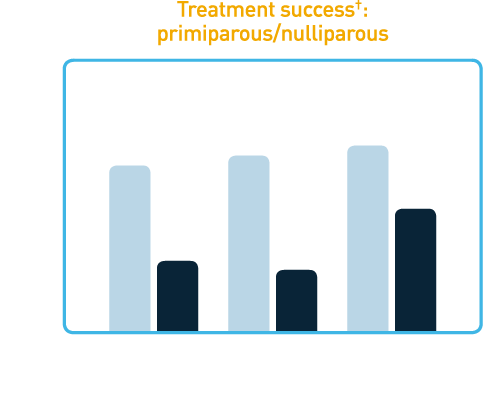
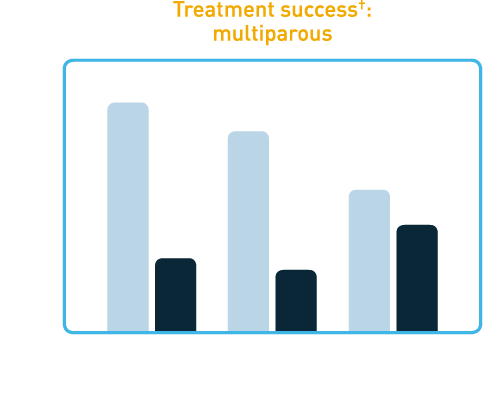

In clinical trials, a single dose of CERVIDIL successfully ripened the cervix in the majority of patients.
†Treatment success was defined as Bishop score increase at 12 hours of ≥3, vaginal delivery within 12 hours, or Bishop score at 12 hours of ≥6. These studies were not designed with the power to show differences in cesarean section rates between CERVIDIL and placebo groups and none were noted.
Shorter Median Time To Delivery1
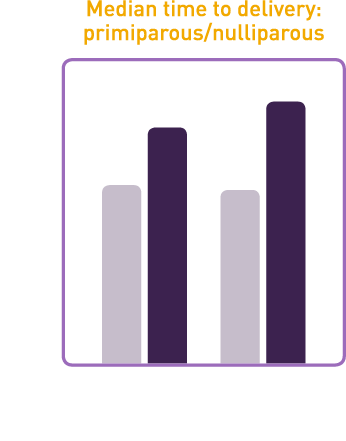
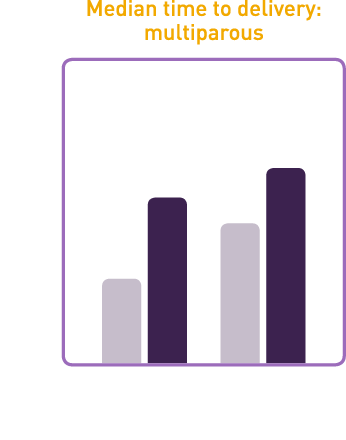
Studies demonstrated that CERVIDIL significantly reduced median time to delivery
- In study 101-103 median time to delivery was 26% shorter for nulliparous and primiparous women and 50% shorter for multiparous women on CERVIDIL vs placebo
- In study 101-801 median time to delivery was 31% shorter for nulliparous and primiparous women and 24% shorter for multiparous women on CERVIDIL vs placebo
Reduced Median Time
to Onset of Labor1
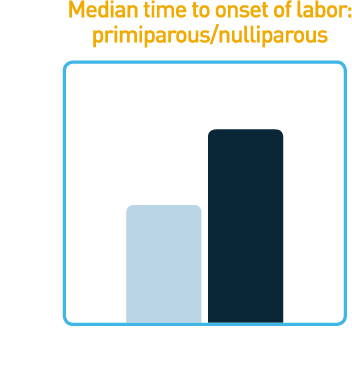
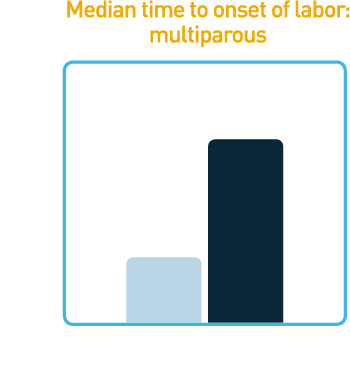
Studies showed that CERVIDIL significantly reduced median time to onset of labor
- Median time to onset of labor of 12 hours was observed in primiparous and nulliparous women
- Multiparous women demonstrated reduced median time to onset of labor of 6.9 hours